Background: Clonal hematopoiesis (CH) is an age-related condition in which somatic mutations can be detected in the blood of healthy individuals. In the non-transplant setting, CH is associated with an elevated risk of developing hematologic malignancy and an increased risk of non-hematologic outcomes due to altered inflammatory signaling. During hematopoietic cell transplantation (HCT), CH in older donors can engraft in recipients and could therefore influence outcomes through effects on graft immunologic function or by causing donor cell leukemia. A definitive link between donor CH and recipient outcomes has not been established. We therefore evaluated the impact of CH in donors aged 40 years or older on recipient clinical outcomes in 1727 donor-recipient pairs.
Methods: We performed targeted, error-suppressed sequencing of 46 genes on 1727 samples from donors age 40 and older. We defined CH as pathogenic mutations at variant allele fraction (VAF) 0.005 or greater. Median donor age was 51 (range 40-80) and median recipient age was 55 (range 1-78). There were 889 matched related donors (51.5%), 454 haploidentical donors (26.3%), 273 matched unrelated donors (15.8%), 71 mismatched unrelated donors (4.1%), and 38 mismatched related donors (2.2%). 929 recipients (53.8%) had myeloid malignancies, 718 (41.6%) had lymphoid malignancies, and 80 (4.6%) had non-malignant diseases. 1022 (59.2%) recipients received peripheral blood stem cell products and 703 (40.7%) received bone marrow. 672 recipients (38.9%) received post-transplant cyclophosphamide (PTCy) for graft versus host disease (GVHD) prophylaxis. Median follow-up for survivors was 6.0 years.
Results: We identified CH in 388 of 1727 (22.5%) donor samples. Mutations in DNMT3A were most common (302 mutations in 253 donors), followed by TET2 (96 mutations in 89 donors), ASXL1 (n=22) and PPM1D (n=14). No other genes were mutated in more than 10 donors (0.5%). The presence of donor CH was independently associated with older donor age, but not with donor sex, donor-recipient relatedness, graft source, or recipient disease.
The presence of donor CH at VAF 0.01 or greater was associated with improved progression-free survival (PFS) in a multivariable model that included donor and recipient age, HCT-CI, disease category, Disease Risk Index score, conditioning intensity, and donor type (HR for death or relapse 0.81, 95% CI 0.68-0.97, P=0.019). This effect was driven by DNMT3A mutations, which were independently associated with improved overall survival (HR for death 0.75; 0.59-0.95, P=0.016) and reduced risk of relapse (sHR 0.76; 0.59-0.97, P=0.029) in the same model. CH involving other gene mutations, including TET2 and genes other than DNMT3A/TET2, was not significantly associated with any recipient outcome. Smaller clones (VAF 0.005-0.01) had no effect on any outcome.
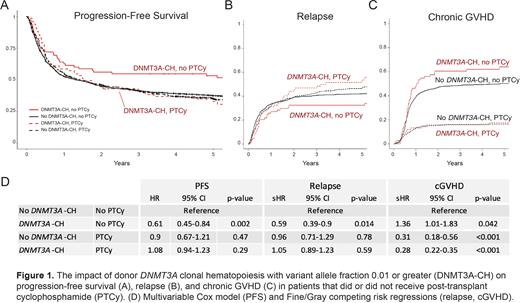
The association between donor DNMT3A-CH and recipient outcomes was limited to those who did not receive PTCy for GVHD prophylaxis (Figure 1A-C). In the model described above, donor DNMT3A-CH in the absence of PTCy was independently associated with improved PFS (HR 0.61; 0.45-0.84, P=0.002), reduced risk of relapse (sHR 0.59; 0.39-0.9, P=0.014) and an elevated risk of chronic GVHD (sHR 1.36; 1.01-1.83, P=0.042) compared to those without DNMT3A-CH. In recipients who received PTCy, there was no significant effect of donor DNMT3A-CH on PFS, relapse, or cGVHD (1D).
Eight recipients developed donor cell leukemia (DCL), for a cumulative incidence of 0.7% at 10 years. In seven of these cases, we identified gene mutations in the corresponding donor products that matched the mutations found in the subsequent DCL, including 2 with TP53 mutations, 3 with splicing factor mutations, and 2 with germline DDX41 mutations that were present in both donor and recipient. No recipients who received products with sole DNMT3A-CH developed DCL.
Conclusions: In HCT donors age 40 or older, the presence of DNMT3A clonal hematopoiesis at VAF >/= 0.01 is independently associated with prolonged overall and progression-free survival in transplant recipients. This effect is driven by reduced risk of disease relapse and confined to recipients who do not receive PTCy, suggesting that it is mediated at least in part by effects on donor T cells. The risk of direct evolution of DNMT3A-CH to donor cell leukemia is low, and most DCLs were traced to atypical donor CH involving MDS-associated genes or germline risk alleles.
Nikiforow:Novartis: Honoraria; Nkarta Therapeutics: Honoraria; Kite/Gilead: Honoraria. DeZern:MEI: Consultancy; Abbvie: Consultancy; Astex: Research Funding; Celgene: Consultancy, Honoraria. Ritz:Rheos Medicines: Consultancy; Infinity Pharmaceuticals: Consultancy; Amgen: Research Funding; Equillium: Research Funding; Kite Pharma: Research Funding; Avrobio: Consultancy; Falcon Therapeutics: Consultancy; TScan Therapeutics: Consultancy; Talaris Therapeutics: Consultancy; LifeVault Bio: Consultancy. Soiffer:VOR Biopharma: Consultancy; Kiadis: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Juno: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; alexion: Consultancy; Be the Match/ National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy; Mana Therapeutics: Consultancy; Precision Bioscience: Consultancy; Cugene: Consultancy; Rheos Therapeutics: Consultancy. Lindsley:Jazz Pharmaceuticals: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; MedImmune: Research Funding; Bluebird Bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal